Which of the Following Is a Statement of Hess's Law
If a reaction is carried out in a series of steps the delta H for the reaction will equal the product of the enthalpy changes for the individual steps. Kryger 21 9 months ago.
If a reaction is carried out in a series of steps the Δ H for the reaction will equal the sum of the enthalpy changes for the individual steps.

. A If a reaction is carried out in a series of steps the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. Which of the following is a statement of hesss law a. Which of the following is a statement of Hesss law A If a reaction is carried.
The delta H for a process in the forward direction is equal to the delta H for the process in the reverse direction. Which of the following is a statement of Hesss law A If a reaction is carried. School Sorsogon State College.
The Hesss law states that when reactants are converted to products the change in enthalpy is the same whether the. Which of the following is a statement of Hesss law. A if a reaction carried out in a series of steps the delta H for the reaction will equal the sum of the enthalpy changes for the individual steps.
The enthalpy of reaction is exothermic if all the intermediate steps are exothermic. Which of the following is a statement of Hesss law. Group of answer choicesIf a reaction is carried out in a series of steps the ΔH for the reaction will equal the sum of the enthalpy changes for the individual stepsIf a reaction is carried out in a series of steps the ΔH for the reaction will equal the product of the enthalpy changes for the.
Which of the following is a statement of Hesss law. The enthalpy of reaction is independent of the reaction path. Hesss Law of Constant Heat Summation or just Hesss Law states that regardless of the multiple stages or steps of a reaction the total enthalpy change for the reaction is the sum of all changes.
Which of the following is a statement of hesss law. Attashe74 19 9 months ago. The enthalpy of reaction is determined from temperature changes in the reaction.
Which of the following is a statement of Hesss law. A If a reaction is carried out in a series of steps the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. Up to 25 cash back Which of the following is a statement of Hesss law.
Pages 5 This preview shows page 2 - 4 out of 5 pages. Which of the following is a statement of Hesss law. B If a reaction is carried out in a series of steps the ΔH for the reaction will equal the product of the enthalpy changes for the individual steps.
Pages 9 This preview shows page 5 - 7 out of 9 pages. Which of the following is a statement of Hesss law. According to Hesss law of constant heat summation the heat absorbed or evolved in a given chemical equation is the same whether the.
The ΔH for a process in the forward direction is equal in magnitude and opposite in sign to the ΔH for the process in the reverse direction. B If a reaction is carried out in a series of steps the ΔH for the reaction will equal the product of the enthalpy changes for the individual steps. Chemistry questions and answers.
Which of the following is a statement of hesss law a. Which of the following is a statement of Hesss law. If a reaction is carried out in a series of steps the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps.
Explain why not all collisions between reactant. This law is a manifestation that enthalpy is a state function. The Δ H for a process in the forward direction is equal to the Δ H for the process in the reverse direction.
C The ΔH for a process in the forward direction is equal in magnitude and opposite in sign to the ΔH. According to this law the chemical equation can be treated as ordinary algebraic expression and can be added or subtracted to yield the required equation. C The ΔH for a process in the forward direction.
Course Title CHM 113. A If a reaction is carried out in a series of steps the AH for the reaction will equal the. Course Title EAD GE 11-2018.
Which of the following is a statement of hesss lawaif a reaction carried out in a series of steps the delta H for the reaction will equal the sum of the enthalpy changes for the individual stepsbif a reaction carried out in a series of steps the delta H for the reaction will equal the product of the enthalpy changes for the individual stepscthe delta. Which of the following is a statement of Hesss law. B If a reaction is carried out in a series of steps the ΔH for the reaction will equal the product of the enthalpy changes for the individual steps.
What is Hess law explain with example. Hesss Law of Constant Heat Summation or just Hesss Law states that regardless of the multiple stages or steps of a reaction the total enthalpy change for the reaction is the sum of all changes. According to Hesss law of constant heat summation the heat absorbed or evolved in a given chemical equation is the same whether the process occurs in one step or several steps.
You might be interested in. A If a reaction is carried out in a series of steps the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. The enthalpy of reaction does not depend on the steps taken in the reaction.
School Arizona State University.

Hess S Law Equation Examples Chemtalk

Solved Which Of The Following Statements Best Describes Chegg Com
![]()
Hess S Law Statement Illustration Application Problems Read Chemistry

Hess S Law Of Heat Of Summation Is Based On Law Of Conservation Of Energy It Has Found Significance In Deriving Heats Of Many Reactions Which Either Do Not Take Place Or If

21 Use Hess S Law To Calculate The Enthalpy Change For The Reaction Wo3 S 3h2 G W S 3h20 G Eqn 1 2w S 302 G Homeworklib

Solved Which Of The Following Is A Statement Of Hess S Law Chegg Com
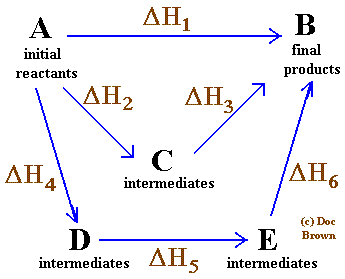
Hess Law Statement Definition Applications Forms Examples

Hess S Law Constant Heat Summation Definition Equation Formula Applications

Solved Questiono Point Which Of The Following Statements Chegg Com
Hess Law Chemistry Problems Enthalpy Change Constant Heat Of Summation Youtube

Solved Which Of The Following Is A Statement Of Hess S Law Ifa Reaction Is Carried Out In A Series Of Steps The Ah For The Reaction Will Equal The Product Of The Enthalpy

Hess S Law Statement Equation Examples What S Insight
What Does Hess S Law State Lisbdnet Com

Solved Which Of The Following Is A Statement Of Hess S Law Chegg Com

Which Of The Following Is A Statement Of Hess S Law Brainly Com

Solved 24 Which Of The Following Is A Statement Of Hess S Chegg Com

Solved 21 Which Of The Following Is A Statement Of Hess S Chegg Com

Energetics Ib Topics 5 15 Part 2 Calculating H Via Bond Enthalpies Hess S Law Above Thermit Rxn Ppt Download

Solved Question 8 Which Statement About Hess S Law Is True Chegg Com

Comments
Post a Comment